
Researchers at the Agile BioFoundry (ABF) and the University of California, San Diego (UCSD) have developed an approach that could help develop new genetic tools to advance the field of algae biomanufacturing.
The approach is essentially a pipeline that can generate the building blocks needed to develop synthetic genetic tools for algae biomanufacturing. Algae can be engineered to sustainably generate a variety of bioproducts that are traditionally made from petroleum. But a lack of sophisticated genetic tools available for engineering algae has limited biomanufacturing efforts.
The pipeline, published in ACS Synthetic Biology, is the result of a collaboration between the ABF and UCSD that aimed to further develop algae as a biomanufacturing platform.
Researchers used a synthetic biology approach to develop advanced genetic tools for algae biomanufacturing, specifically focusing on synthetic promoters, which help control gene expression. They analyzed DNA promoters from six different algae species to identify conserved cis-regulatory elements (CREs), which might boost gene activity.
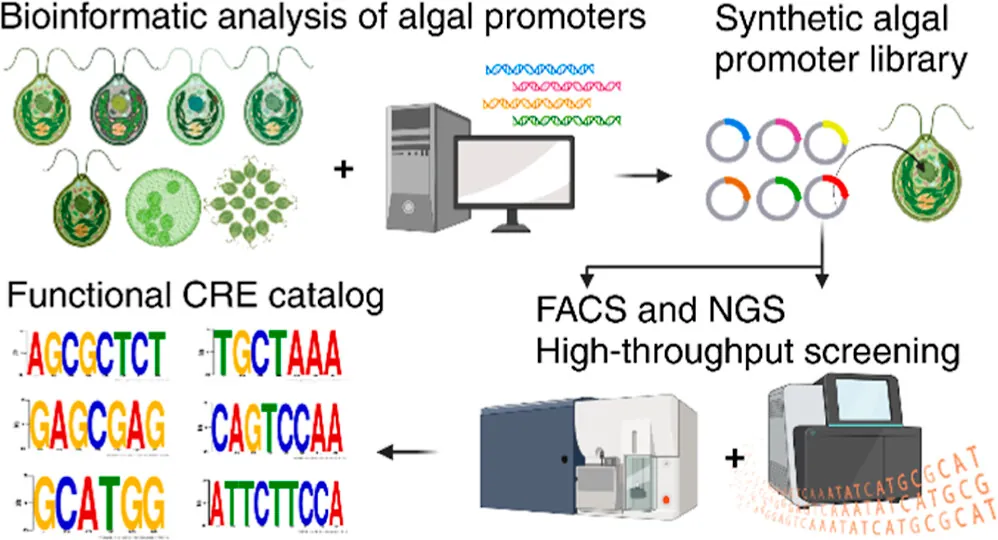
Using bioinformatic algorithms, the researchers predicted over 1,500 CREs and built two synthetic algal promoter libraries. They tested each CRE in different combinations, which ended up generating over 4,500 distinct synthetic promoters.
A number of these promoters were tested in algae for their impact on gene expression. Many were found to perform at a higher level than baseline synthetic promoters, and some even better than top native promoters.
Not only did this work identify a catalog of CREs that can help build high-performing synthetic algal promoters, it also established a pipeline for developing innovative synthetic genetic tools for any type of algae.
“It’s pretty clear that this process is working really well,” said Stephen Mayfield, project co-lead and Distinguished Professor of Molecular Biology at UCSD. “We can make a lot of very potent promoters now.”
Mayfield said the efficient collaboration with the ABF helped the project run smoothly.
“The ABF team cloned all of the synthetic promoters into bacteria, and then sequenced and cloned them after they were confirmed to be working well. When we got them, all we had to do was amplify the DNA and transform it into algae,” Mayfield said. “It worked really seamlessly for us.”
The promoters identified in this work are now available to the broader algae research community. The researchers hope that the foundational work done in this project will help take algae biotechnology to the next level.
The ABF is supported by the U.S. Department of Energy’s Bioenergy Technologies Office (BETO).
ABF Capabilities used in this collaboration: